DIABETES
Pharmacological management of type 2 diabetes
Early initiation of pharmacological therapy is associated with improved glycaemic control and reduced long-term complications in type 2 diabetes
February 9, 2018
-
Type 2 diabetes is a chronic metabolic disorder that results from either insulin resistance and/or insulin deficiency. This type of diabetes represents 90% of all diabetes cases and the current epidemic in type 2 diabetes is largely seen in an ageing population and with obesity.
The focus in managing type 2 diabetes is on putting the patient at the centre of care and the requirement of constant monitoring and treatment throughout the patient’s life. The treatment involves several aspects like self-care measures, lifestyle changes and in most cases alteration of medications.
Prevalence
Approximately 422 million people worldwide have diabetes, which translates into almost one in every 11 people having the condition. In Ireland 3.3% of women and 4.3% of men had diabetes in 1980. This rose to 5.1% of women and 7.3% of men in 2015.1
Screening and diagnosis
The ICGP Guide to integrated type 2 diabetes care2 recommends that testing for diabetes should be considered in all adults who are overweight (BMI ≥ 25kg/m2) and who have one or more additional risk factors as listed below:
• Physical inactivity
• First-degree relative with diabetes
• Are hypertensive (≥ 140/90mmHg) or on therapy for hypertension
• Dyslipidaemia: HDL < 0.9 and/or triglycerides > 2.82
• Have established arterial disease (IHD, CVA, PVD)
• High-risk ethnicity (eg. African, Asian, Hispanic, etc.)
• Members of the Travelling Community
• Have delivered a baby weighing > 4.1kg or have a history of gestational diabetes
• On previous testing had impaired glucose tolerance (IGT) or impaired fasting glucose (IFG)
• Have other clinical conditions associated with insulin resistance (eg. polycystic ovary syndrome, acanthosis nigricans, long-term steroid use or severe obesity).
In the absence of the above additional risk factors, the ICGP guide recommends that overweight adults age 45 and older get screened for type 2 diabetes at least every three years.2
In 2011, the WHO approved HbA1c as a diagnostic test for diabetes. To aid screening and early detection of diabetes, HbA1c can now also be used to diagnose pre-diabetes (see Table 1).
Pathophysiology
Insulin insensitivity in muscle and liver associated with β-cell failure represent the crucial defects in diabetes. In addition to muscle, liver, and β-cells, α-cells (hyperglucagonaemia), adipocytes (accelerated lipolysis), gastrointestinal tract (incretin deficiency/resistance), kidney (increased glucose reabsorption), and brain (insulin resistance and neurotransmitter dysregulation) play important roles in the development of glucose intolerance and later type 2 diabetes.
Management strategies for diabetes
The management approach below is based on guidelines from the American Diabetes Association (ADA),3 the National Institute for Health and Care Excellence (NICE)4 and the ICGP’s most recent guidelines.2
As diabetes is seen across the lifespan, co-ordination between primary care teams and secondary care providers as patients transition through different stages of life is crucial. An effective framework for improving the quality of diabetes care needs to be in place in collaboration with multidisciplinary teams. The main objectives for primary care providers should be to prioritise prevention, diagnosis, initial management and continuing care:
•Primary care teams should manage timely and appropriate intensification of lifestyle and/or pharmacological therapy for patients who have not achieved beneficial levels of glucose, blood pressure, or lipid control
•Optimal diabetes management requires an organised, systematic approach and involves a co-ordinated team of dedicated healthcare professionals
•Primary care providers should focus on treatment intensification when treatment goals are not met. This has been associated with improvement in A1c, hypertension, and hyperlipidaemia
•Patient adherence should be addressed to avoid barriers such as complexity, multiple daily dosing, cost and side-effects. Treatment adherence should be improved by simplifying a complex treatment regimen.
The 2016 ICGP Guide to integrated type 2 diabetes care2 provides a good national integrated model of care to illustrate the care pathway for people with type 2 diabetes (see Table 2).
Approach to management of hyperglycaemia
Lifestyle optimisation
Lifestyle and nutrition counselling is essential for patients with prediabetes or new-onset diabetes to slow the progression of type 2 diabetes.5 Clinical trials have shown that calorie restriction is typically the primary method for weight loss. Patients who are overweight or obese could see multiple health benefits, including significant improvement in HbA1c, systolic and diastolic blood pressure, high-density lipoprotein cholesterol (HDL-C), and triglycerides, by losing just 5%-10% of body weight.6
In addition to calorie intake restriction, the American Association of Clinical Endocrinologists/American College of Endocrinology (AACE/ACE) guidelines recommend a diet largely focused on vegetables and polyunsaturated fatty acids, such as those found in fatty fish (eg. salmon, mackerel, and trout) and monounsaturated fats (eg. olive oil). Trans-saturated fats should be avoided.7 See also Table 3 for approach to management of hyperglycaemia.
Foundations of care
Individuals with diabetes are advised on self-management and encouraged to follow a healthy eating pattern with appropriate portion sizes to achieve and maintain body weight goals, individualised glycaemic targets, blood pressure control and lipid goals.
All adults with diabetes are advised to undertake at least 150 minutes of moderate-intensity aerobic activity over at least three days/week or resistance training at least twice weekly. Smoking cessation is encouraged in all patients with type 2 diabetes.
Looking beyond glucose control
People with type 2 diabetes and hypertension should be treated to a systolic blood pressure goal of ≤ 140mmHg and lower targets may be appropriate for certain individuals.
As a primary prevention strategy in people with type 2 diabetes, in those at increased cardiovascular risk (10-year risk > 10%) aspirin therapy of 75mg/day should be considered. Depending on risk, in most patients with diabetes aged 40 and above, remember to use moderate or high dose statins.8
ADA/NICE recommends an HbA1c ≥ 48mmol/mol (6.5%) as the threshold for initiating or up-titrating therapy in general, while ≥ 58mmol/mol (7.5%) remains the trigger for triple therapy.9
Pharmacological therapy
Early initiation of pharmacologic therapy is associated with improved glycaemic control and reduced long-term complications in type 2 diabetes.
The initial treatment is guided by the level of HbA1c at diagnosis, the presence of osmotic symptoms, evidence of catabolic state, and the presence of chronic diabetes complications that may preclude the use of a particular therapeutic agent.
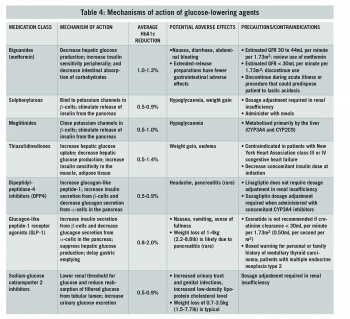
In addition, a patient’s age, body weight, convenience of administration, and impact on work-related issues (such as driving motor vehicles) also play a crucial role in determining the order of medications used. The efficacy and side-effect profile of each drug in the individual patient is also taken into account. See mechanisms of action of glucose-lowering agents in Table 4.
Metformin
Metformin is considered the agent of first-line for treatment of type 2 diabetes in the absence of contraindications. Metformin lowers basal and postprandial plasma glucose levels. Metformin alters the composition of gut microbiota and activates mucosal AMP-activated protein-kinase (AMPK) that maintains the integrity of the intestinal barrier. Metformin in combination with the activation of AMPK decreases hepatic gluconeogenesis. It also decreases intestinal absorption of glucose and improves insulin sensitivity by increasing peripheral glucose utilisation.10
Metformin reduces fasting blood glucose by approximately 20% and HbA1c by 1.5%. It can be used in combination with sulphonylureas, glinides, alpha-glucosidase inhibitors, insulin, thiazolidinediones (TZD), glucagon-like peptide-1 receptor agonists (GLP1), dipeptidylpeptidase-4 inhibitors (DPP4), and sodium-glucose co-transporter 2 inhibitors (SGLT2).
Metformin is contraindicated in patients with factors that predispose to lactic acidosis, such as renal impairment, concomitant liver disease or excessive alcohol intake, unstable or acute heart failure and hypoxia.10
Insulin secretagogues: sulphonylureas and meglitinides
Sulphonylureas are commonly used as second-line agents in patients with type 2 diabetes, They can act as an alternative first-line treatment if the patient cannot tolerate metformin and if the patient is not overweight. Sulphonylureas can also be added to metformin if glycaemic control is inadequate. Sulphonylureas stimulate pancreatic beta cells to release insulin.
The glucose-lowering effect is said to be high for sulphonylureas. The main side-effects are loss of efficacy due to beta cell failure, weight gain and hypoglycaemia.11
Thiazolidinediones
NICE recommends that thiazolidinediones (TZDs) should be considered as second-line therapy, in addition to metformin, if the risk of hypoglycaemia with sulphonylureas would be unacceptable.
TZDs increase insulin sensitivity by acting on muscle and adipose tissue to increase glucose utilisation and decrease glucose production in the liver.
TZDs act as insulin sensitisers; thus, they work in the presence of insulin. They must be taken for 12-16 weeks to achieve maximal effect. TZDs are associated with an increased fracture risk and in some patients may lead to heart failure.11 There is also a possible increased risk of bladder cancer with use of pioglitazone.11
Weight gain is the result of fluid retention and the activation of PPAR-gamma in the central nervous system (which increases feeding) and the up-regulation of genes that facilitate adipocyte lipid storage.
Incretin-based therapy
The incretin agents (GLP1 and GIP), secreted by intestine L cells, increase insulin secretion and inhibit glucagon in response to nutrient inputs. The glucoregulatory effects of incretins are the basis for treatment with inhibitors of DPP4 in patients with type 2 diabetes. Agents that inhibit DPP4, an enzyme that rapidly inactivates GLP1, increase active levels of these hormones and, in doing so, improve islet function and glycaemic control in type 2 diabetes.11
Dipeptidyl peptidase-4 inhibitors
DPP-4 inhibitors (eg. sitagliptin, saxagliptin, linagliptin, vildagliptin) are a class of drugs that prolong the action of incretin hormones. DPP-4 degrades numerous biologically active peptides, including the endogenous incretins GLP-1 and glucose-dependent insulinotropic polypeptide (GIP). DPP-4 inhibitors can be used as a monotherapy or in combination with metformin or a TZD. They are given once daily and are weight neutral.
GLP-1 agonists (ie. exenatide, liraglutide, dulaglutide)
GLP1 is secreted in response to food intake and stimulates insulin release, reduce glucagon, and slow gastric emptying. GLP-1 agonists are easy to use, as they are characterised by fixed doses and flexibility in time of administration (except exenatide).
The glucose-dependent mechanism of action, and the resultant lack of hypoglycaemia is a major advantage which increases the safety of these drugs, while maintaining efficacy.12 Weight loss with liraglutide and exenatide, and lack of weight gain with DPP-4 inhibitors is another factor which encourages use of this class of drugs. GLP-1 receptor agonists aid weight loss, and liraglutide was recently licensed for individuals without diabetes as a weight loss treatment.13 A common side-effect of GLP-1 receptor agonists is nausea, which is usually temporary and disappears around two weeks after treatment initiation. In addition, GLP-1 receptors also increase satiety and augment weight loss.14 At present, GLP-1 receptor agonists are only available in an injectable form.
The lack of other major side-effects such as oedema and GI disturbance with DPP-4 inhibitors is another advantage. The incretin-based therapies can be used in mild to moderate renal failure with appropriate dose adjustments, and can be given to elderly patients.
Newer therapies targeting renal glucose handling
Sodium glucose cotransporter-2 inhibitors
SGLT2 inhibitors (eg. dapagliflozin, canagliflozin, empagliflozin), the newest antihyperglycaemic drug class, have a novel mechanism of action. Instead of inhibiting hormones and enzymes involved in the digestive process, this new drug class targets the SGLT2 protein, which is located on the proximal renal tubules. By inhibiting SGLT2, the renal threshold for glucose is reduced, renal glucose reabsorption is blocked, and glycosuria is increased.
The change in the renal glucose threshold is likely to be behind the low rate of hypoglycaemia seen with SGLT2 inhibitors.
The mechanism of action is insulin-independent, which makes SGLT2 inhibitors complementary to other glucose-lowering treatments. Average HbA1c reduction afforded by SGLT2 inhibitors when used as monotherapy or as an add-on to metformin is 0.32% to 1.17%. As with other type 2 diabetes therapies, greater HbA1c reduction is observed with higher baseline levels. Average weight loss recorded in clinical trials was 1.5-3kg compared with placebo; however, weight loss tends to plateau, and some patients regain lost weight.
Adverse events include polyuria, genitourinary infections, hypotension, bone fractures, and diabetic ketoacidosis. With regard to the latter, the American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology (ACE) convened a conference in October 2015 to review the clinical data. An expert consensus statement was issued noting that the incidence of diabetic ketoacidosis to be ‘infrequent’, requiring no change in recommendations.14
Newer insulins
Longer-acting insulins have been developed to provide a more stable basal insulin profile over a 24-hour period. The action of insulin can be delayed by increasing its molecular size and slowing its absorption. Degludec is formed of hexameric chains that slowly separate, releasing the active monomer that can then be absorbed. In clinical trials, in patients with type 2 diabetes, it was non-inferior to glargine and had lower levels of hypoglycaemic episodes, particularly at night (nocturnal hypoglycaemia).14
The long half-life of degludec compared with traditional, long-acting insulins means that patients can be much more flexible about the timing of their basal insulin doses, without compromising their glycaemic control.
This may be particularly useful for people with erratic lifestyles. Glargine U300 is a more concentrated form of insulin glargine available as 300 units/ml in a pre-filled pen. U300 also has a longer half-life compared with glargine. In trials, it was associated with fewer nocturnal hypoglycaemic episodes than glargine, with no change in the overall glycaemic control.14
Ensuring adherence to treatment
The prevalence of type 2 diabetes is increasing in Ireland due to the ageing population and increase in obesity. A multidisciplinary approach for the management of diabetes includes involving the patient. Successful treatment of type 2 diabetes is often complicated by the inevitably progressive nature of the condition and the need to balance target blood glucose levels against an increased risk of treatment-related adverse effects such as hypoglycaemia and weight gain.
All patients with diabetes need full initial assessment and regular review to ensure that they are achieving their target HbA1c. They should be monitored for CV risk factors and microvascular complications. A lifestyle intervention programme to promote weight loss and increase activity levels should be included as part of diabetes management. Adherence to treatment is often compromised by the fear of hypoglycaemia and weight gain, and therefore patient education is a vital aspect of management. Most patients require combination glucose-lowering agents and many patients will require insulin.
References
- Tracey et al. Epidemiology of diabetes and complications among adults in the Republic of Ireland 1998-2015: a systematic review and meta-analysis. BMC Public Health (2016) 16:132
- ICGP, DOHC, HSE. A Practical Guide to Integrated Type 2 Diabetes Care. Dr Velma Harkins, 2016. https://www.icgp.ie/go/library/catalogue/item/B5C683DA-ECE8-2264-DD43F57101FDA2A6
- ADA Standards of medical care in diabetes, 2018. Accessible on https://professional.diabetes.org/content-page/standards-medical-care-diabetes
- NICE Type 2 diabetes in adults: management. NICE guideline [NG28] 2015, Last updated: May 2017. https://www.nice.org.uk/guidance/ng28
- Tuomilehto J, Lindström J, Eriksson JG et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344(18):1343-1350
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2017 executive summary. Endocr Pract. 2017; 23:207-238
- Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care. 2007; 30:1374-1383
- King P, Peacock I & Donnelly R. The UK Prospective Diabetes Study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol 1999; 48(5):643-648
- American Diabetes Association. Implications of the United Kingdom Prospective Diabetes Study. Diabetes Care 2000; 23(1):S27-31
- Campbell IW. The UK Prospective Diabetes Study (UKPDS): Its legacy for type 2 diabetes management. Prim Care Cardiovasc J, 2009; 2(1):48-49
- Tran L, Zielinski A, Roach AH et al. The pharmacologic treatment of type 2 diabetes: oral medications. Ann Pharmacotherapy, 2015; 49(5):540-556
- Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context. 2015; 4:212283
- Pi-Sunnier X, Satrap A, Guijosa K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. NEJM 2015; 373(1): 11-22
- Handelsman Y, Henry RR, Bloomgarden ZT, et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of SGLT-2 inhibitors and diabetic ketoacidosis. Endocr Pract. 2016; 22:753-762