CANCER
Cancer supportive care: delivering clinical and cost efficiencies
With the number of cancer cases rising, so too are the costs associated with treatment and the side-effects of treatment
May 5, 2014
-
The burden of cancer is growing, and the disease is becoming a major economic expenditure for all developed countries. The World Cancer Report 2014 predicts that the number of new cancer cases each year will rise by 75% to 25 million over the next two decades.1 The National Cancer Registry (NCR) projects that the incidence of cancer in Ireland will double by 2040; a growing and ageing population will be the main factors driving this increase, with the number of cancer cases diagnosed each year rising from 28,000 to 60,000.2
Rising treatment costs
The spiralling costs of this cancer burden are damaging the economies of even the richest countries and placing impossible strains on healthcare systems.3 Luengo-Fernandez et al estimated that cancer-related health costs in Ireland amounted to €619 million in 2009: €417 million on inpatient care; €127 million on drugs; and €32 million on primary care. In all, cancer consumed 4% of total health spending in Ireland.3
As cancer cases continue to rise, the NCR has warned that so too will treatment numbers and associated costs. Patients having cancer-directed surgery are projected to increase by 50-55% between 2010 and 2025, the number having chemotherapy by 42-48% and the number having radiotherapy by 32-35%. These changes, combined with increasing survival, will inevitably increase the burden on cancer services.2
Supportive care in optimising treatment outcomes
Often the treatments used to manage cancer can produce a wide range of adverse reactions and toxicities. The adverse effects of chemotherapy, for example, range from mild, such as fatigue, to life threatening, as in febrile neutropenia. Other common treatment-induced and cancer-related symptoms include anaemia, bone loss, pain, nausea and vomiting.
Cancer patients experiencing treatment-related adverse events have substantially higher healthcare costs, which increase with the number of adverse events reported. Better management of these treatment-induced symptoms may represent an opportunity to significantly reduce costs and improve clinical outcomes.4
Anticipating and managing these adverse events can help to minimise them and provide the best possible experience for the person receiving cancer therapy, and may help prevent dose reduction or discontinuation of therapy.
Haematological adverse effects
Chemotherapy-related haematological adverse events are relatively common among patients receiving cancer treatments, including neutropenia, thrombocytopenia, and anaemia. These adverse events result in a substantial clinical and economic burden on patients, payers, caregivers and society in general. Liou et al estimated the cost of neutropenia ranged from approximately €1,900 per outpatient episode to €36,300 per febrile neutropenia hospitalisation (although, for countries outside the US, the cost of neutropenia appeared to be lower). Treatment of thrombocytopenia cost on average €3,300, and anaemia costs ranged from €16,500 to €68,000 per year.5
Neutropenia
Chemotherapy-induced neutropenia is a major risk factor for infection-related morbidity and mortality and also a significant dose-limiting toxicity in cancer treatment.6 Approximately 7-22% of cancer patients who are treated with chemotherapy require hospitalisation for neutropenia.7,8,9,10
Febrile neutropenia
Febrile neutropenia (FN), or the presence of fever in a patient with neutropenia, is a medical emergency and is associated with a substantial increased risk for morbidity, mortality, and hospitalisation, which ultimately increase the cost of cancer care.11 Even previously stable patients can quickly become haemodynamically unstable and critically ill. Prompt assessment and interventions are necessary to reduce morbidity and mortality.
Timely initiation of therapy with prescribed IV fluid and broad-spectrum antibiotics administered within 60 minutes of the start of the febrile episode lead to the best outcomes for a patient with febrile neutropenia.12 High-risk patients require hospitalisation for treatment. Patients meeting low-risk criteria may receive outpatient oral or parenteral antibiotic therapy.13
The Multinational Association for Supportive Care in Cancer (MASCC) Risk Index for febrile neutropenia14 (See Figure 1) and the use of granulocyte-colony stimulating factor (G-CSF; eg. lipegfilgrastim, filgrastim, lenograstim and pegfilgrastim) for prevention have been the major advances over the past 30 years.15
The use of G-CSF has been widely accepted into routine clinical practice to reduce the risk of FN associated with cytotoxic chemotherapy. Prophylactic use of G-CSF reduces the incidence of FN by about 50%, reduces mortality, without adversely affecting patient quality of life, and is cost effective.16
Short-acting G-CSFs (eg. filgrastim) require daily subcutaneous injections during each chemotherapy cycle. Newer, long-acting G-CSFs (eg. lipegfilgrastim, pegfilgrastim) facilitate once-per-cycle dosing. Bondarenko et al found that the incidence and duration of severe neutropenia in patients who received lipegfilgrastim was similar to or lower than that of patients who received pegfilgrastim.17 Researchers concluded that the introduction of new treatments might help improve the cost efficacy of managing cancer patients receiving chemotherapy.
Both long-acting and short-acting G-CSF can be used for patients with a 20% or greater risk of developing FN. By strict definitions, patients with a calculated risk <20% are not eligible for G-CSF but it has been suggested that the criteria should be expanded for use of G-CSF as effective primary prophylaxis to include those at lower risk.15
With regard to duration of G-CSF therapy, the highest incidence of FN and largest benefit of G-CSF during the first cycles of chemotherapy has led to questions about the effectiveness of continued use of G-CSF throughout later cycles of chemotherapy.
However, Aarts et al found that when the use of prophylactic G-CSF to reduce FN was decreased in patients receiving chemotherapy for breast cancer, it resulted in an increase of more than five-fold in FN episodes and an early end to the clinical trial.18
The authors concluded that primary G-CSF prophylaxis should not be limited to the first two chemotherapy cycles because of an unacceptable high FN rate.
“In fact, this rate is as high as that reported in previous studies of TAC (docetaxel, doxorubicin, and cyclophosphamide) chemotherapy without primary G-CSF prophylaxis…Therefore, we recommend primary G-CSF prophylaxis throughout all chemotherapy cycles in patients at risk for FN,” they wrote.
Anaemia
Anaemia is a common side effect of cancer and cancer therapy (30% to 90% of cancer patients).19 Its prevalence varies with tumour type, stage, and therapy used.20 The negative impact of anaemia symptoms, such as fatigue, on patient quality of life is substantial.21 In addition, anaemia may compromise patients’ tolerance of treatments, resulting in the need to reduce the duration or intensity of those treatments.22
Anaemia can be corrected through either treating the underlying cause or providing supportive care through red blood count (RBC) transfusion or administration of erythropoiesis-stimulating agents (ESAs), such as epoetin alfa and darbepoetin alfa, that stimulate RBC production, with or without iron supplementation.
Popularity of ESAs reached a peak in 2003 to 2004, however, this changed dramatically after evidence emerged of increased venous thromboembolism (VTE) and mortality in certain patient groups. As a result, the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for Cancer- and Chemotherapy-Induced Anaemia underwent substantial revisions in 2012.23
As randomised trial data suggest that ESAs may promote tumour growth in an off-target manner, the NCCN recommended that these agents should not be used when the anticipated outcome is cure (eg. primary and adjuvant chemotherapy for malignancies such as early-stage breast cancer and NSCLC, lymphomas and testicular cancer, etc). However, it is not always clear whether a chemotherapy regimen is considered curative, therefore, the options for anaemia management should be: consideration of RBC transfusion, clinical trial enrolment if available, and consideration of ESAs.19
Treatment-related bone loss
Cancer treatments can result in significant bone loss and increased risk of fragility fracture. Chemotherapy, aromatase inhibitors and gonadotropin-releasing hormone (GnRH) analogues can result in an increased rate of bone remodelling and decreased bone mineral density. Prostate cancer patients receiving androgen deprivation therapy may also experience increases in the risk of fracture.24
Evaluation and treatment of cancer treatment-related bone loss should be incorporated into comprehensive cancer care. Early assessment of skeletal health followed by initiation of lifestyle changes, such as increasing vitamin D and calcium intake, and performing weight-bearing exercises regularly, are valuable in the prevention and treatment of osteoporosis.
In addition, those at an increased risk for fracture should be offered bone-modifying agents (BMAs), such as bisphosphonates (inhibitors of bone resorption and increase bone mineral density [BMD] by altering both osteoclast activation and function) and denosumab (a monoclonal antibody directed against the receptor activator of nuclear factor kappa-B ligand), which reduce bone resorption.24
Trials employing the use of bisphosphonates (zoledronate, risedronate, alendronate, ibandronate, clodronate, and pamidronate) in patients receiving aromatase inhibitors or chemotherapy have demonstrated that these agents increase bone mineral density and decrease bone turnover and the risk of fractures.25
Pasterson et al conducted a systematic review of clinical trials and meta-analyses published between 1996 and 2012. They concluded that zoledronate, pamidronate, clodronate, and denosumab are recommended for metastatic breast cancer patients, however, no one agent can be recommended over another.25
The authors also advised that zoledronate or any oral bisphosphonate and denosumab should be considered in primary breast cancer patients who are postmenopausal on aromatase inhibitor therapy and have a high risk of fracture and/or a low bone mineral density, and in premenopausal primary breast cancer patients who become amenorrheic after therapy.
Although a number of clinical trials found that denosumab was superior to zoledronate in delaying the time to first skeletal-related event significantly more in patients with breast or castration-resistant prostate cancer with bone metastasis, the overall survival and progression-free survival were similar. As a result, evidence is insufficient to prove a greater efficacy of one agent over the other.26
According to the American Society of Clinical Oncology (ASCO) and the NCCN, patients with bone metastasis should have zoledronate, pamidronate, or denosumab (with calcium and vitamin D supplementation) added to their chemotherapy regimen if they have an expected survival of three months or longer and have adequate renal function.
It is worth noting that denosumab may be particularly useful in clinical practice for the treatment of patients with gastrointestinal contraindications or side effects with oral bisphosphonates and for patients with malabsorption.27
Nausea and vomiting
Despite advances in medicine over the past few decades, nausea and vomiting continue to be two of the most common and distressing side effects of chemotherapy. A prospective observational study of 143 patients who received a total of 766 cycles of chemotherapy found that prevalence rates of any nausea or vomiting were 37% and 13%, respectively, at 24 hours and 70% and 15% during days two to five.28
Not only can nausea have a negative impact on quality of life, both physical and cognitive functions, it may lead to critical delays in the administration of potentially curative therapy.29
Modern antiemetics can be very effective in controlling nausea, and healthcare professionals have shifted their focus from treating nausea to its aggressive prevention.
An expert international panel was convened in Perugia, Italy, in 2009, to review various issues involving chemotherapy- and radiotherapy-induced nausea and vomiting, and develop consensus statements. The topics addressed by members of the Multinational Association of Supportive Care in Cancer (MASCC) and the European Society for Medical Oncology (ESCO) included antiemetic therapy of multiple-day chemotherapy, high-dose chemotherapy, and rescue antiemetics.30
Other organisations, including ASCO and NCCN, have developed guidelines for antiemetic prevention, and a summary of these recommendations is contained in Table 1.31
It is generally recommended that patients receiving multiple-day cisplatin should receive a 5-HT3 receptor antagonist plus dexamethasone for acute nausea and vomiting, and dexamethasone for delayed nausea and vomiting.30
In addition, a recent meta-analysis of randomised controlled trials comparing palonosetron with other 5-HT3 receptor antagonists revealed that it was significantly more effective in preventing acute and delayed nausea and vomiting for highly emetogenic and moderately emetogenic chemotherapy.32
Controlling nausea and vomiting in patients undergoing high-dose chemotherapy with stem cell support remains a challenge. Standard therapy appears to be a 5-HT3 receptor antagonist with dexamethasone with or without aprepitant (See Table 1).30
Pain control
According to a systematic review of the literature, cancer pain prevalence ranges from 33% in patients after curative treatment to 59% in patients on anticancer treatment and to 64% in patients with metastatic, advanced or terminal phase.33 Factors influencing the development of chronic pain in cancer survivors who have completed treatment include peripheral neuropathy due to chemotherapy, radiation-induced brachial plexopathy, chronic pelvic pain secondary to radiation and postsurgical pain.34
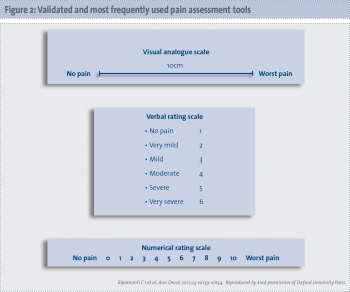
The ESMO issued guidelines in 2012 for the adequate assessment of the patient with pain at any stage of the disease and for the management of cancer pain (See Figure 2).35
According to published guidelines,36,37,38 opioid analgesics are the mainstay of analgesic therapy, come in different formulations (eg. slow-release, transdermal, transmucosal, intranasal, sublingual, and patient-controlled analgesia), and are classified according to their ability to control pain from mild to moderate (codeine, dihydrocodeine, tramadol, dextropropoxyphene; second step of the WHO analgesic ladder) and moderate to severe pain (morphine, methadone, oxycodone, buprenorphine, hydromorphone, fentanyl, heroin, levorphanol, oxymorphone; third step of the WHO analgesic ladder).39
Morphine is considered the gold standard ‘step III’ opioid.36,37 Ideally, two types of formulations are required: normal-release morphine (NRM; for dose titration) and sustained release (for maintenance treatment).
Although the oral route of administration is advocated, patients presenting with severe pain that needs urgent relief should be treated and titrated with parenteral opioids, usually administered by the subcutaneous (sc) or intravenous (iv) route. If given parenterally, the equivalent dose is one-third of the oral medication.
The relative potency ratio of oral to parenteral (sc or iv) morphine might vary according to the circumstances in which morphine is used and among individual patients. When converting from oral to parenteral morphine, the dose should be divided by two or three to get a roughly equianalgesic effect, but upward or downward adjustment of the dose may then be required.40
About 10% of cancer patients have pain that is difficult to manage with oral or parenteral analgesic drugs. Interventional techniques such as nerve blocks and intrathecal drug delivery (spinal or epidural) may allow those patients refractory to all conventional strategies and/or dose limiting analgesic-related side effects to reach pain control when used as unique therapy or, more frequently, in combination with systemic therapy.38
Conclusion
As survival of cancer and in particular metastatic cancer increases, patients have greater exposure to cancer treatments and related toxicity. Supportive care alleviates symptoms and complications of cancer, reduces or prevents toxic effects of treatment, supports communication with patients about their disease and prognosis, allows patients to tolerate and benefit from active therapy more easily, eases emotional burden of patients and caregivers, and helps cancer survivors with psychological and social problems.41
References
- Stewart BW, Wild CP, eds. World Cancer Report 2014. International Agency for Research on Cancer. Lyon, France, 2014.
- Cancer Projections for Ireland 2015-2040. National Cancer Registry Ireland. Cork, 2014.
- Luengo-Fernandez R, Leal J, Gray A, et al. Economic burden of cancer across the European Union: a population-based cost analysis. The Lancet Oncology 2013; 14(12): 1165-1174.
- Hansen RN, Brammer MG, Ramsey SD, et al. The cost of treatment-related adverse events in metastatic breast cancer therapy: Taxanes versus capecitabine. J Clin Oncol 2013; 31 (suppl; abstr e17527).
- Liou SY, Stephens JM, Carpiuc KT, et al. Economic burden of haematological adverse effects in cancer patients: A systematic review. Clin Drug Invest 2007; 27(6): 381-396.
- Aapro MS, Bohlius J, Cameron DA, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 2011; 47(1): 8-32.
- Cullen M, Steven N, Billingham L, et al. Antibacterial prophylaxis after chemotherapy for solid tumors and lymphomas. NEJM 2005; 353(10): 988-98.
- Donohue R. Development and implementation of a risk assessment tool for chemotherapy-induced neutropenia. Oncol Nurs Forum 2006 March; 33(2): 347-52.
- Du XL, Osborne C, Goodwin JS. Population-based assessment of hospitalizations for toxicity from chemotherapy in older women with breast cancer. J Clin Oncol 2002; 20(24): 4636-42
- Hartmann LC, Tschetter LK, Habermann TM, et al. Granulocyte colony-stimulating factor in severe chemotherapy-induced afebrile neutropenia. NEJM 1997; 336(25): 1776-80.
- Kuderer NM, Dale DC, Crawford J, et al. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 2006; 106 (10): 2258-2266.
- Cameron D. Management of chemotherapy-associated febrile neutropenia. Br J Cancer 2009; 101(suppl 1): S18-S22.
- Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2011; 52(4): e56-e93.
- MASCC Risk Index for febrile neutropoenia. http://www.qxmd.com/calculate-online/hematology/febrile-neutropenia-mascc. Accessed online March 30, 2014.
- Klastersky J. A 30-year perspective: Progress in febrile neutropenia in patients with cancer. MASCC/ISOO International Symposium on Supportive Care in Cancer. Presented June 28, 2012.
- Lee S, Knox A, Zeng IS. Primary prophylaxis with granulocyte colony-stimulating factor (GCSF) reduces the incidence of febrile neutropenia in patients with non-Hodgkin lymphoma (NHL) receiving CHOP chemotherapy treatment without adversely affecting their quality of life: cost-benefit and quality of life analysis. Support Care Cancer 2013; 21(3): 841-6.
- Bondarenko I, Gladkov OA, Elsaesser R, et al. Efficacy and safety of lipegfilgrastim versus pegfilgrastim: a randomized, multicenter, active-control phase 3 trial in patients with breast cancer receiving doxorubicin/docetaxel chemotherapy. BMC Cancer 2013; 13: 386 -398.
- Aarts MJ, Peters FP, Mandigers CM, et al. Primary granulocyte colony-stimulating factor prophylaxis during the first two cycles only or throughout all chemotherapy cycles in patients with breast cancer at risk for febrile neutropenia. JCO 2013; 31(34): 4290-4296.
- Rogers GM 3rd, Becker PS, Blinder M, et al. Cancer- and chemotherapy-induced anemia. J Natl Compr Canc Netw 2012; 10(5): 628-653.
- Harrison LB, Shasha D, Homel P. Prevalence of anemia in cancer patients undergoing radiotherapy: prognostic significance and treatment. Oncology 2002; 63(suppl 2): 11-18.
- Cella D. The effects of anemia and anemia treatment on the quality of life of people with cancer. Oncology (Williston Park) 2002; 16(suppl 10): 125-132.
- Littlewood T, Mandelli F. The effects of anemia in hematologic malignancies: more than a symptom. Semin Oncol 2002; 29(suppl 8): 40-44.
- Recent Updates to NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Accessed http://www.nccn.org/
- Khan A, Khan N. Cancer Treatment Related Bone Loss. Current Oncology 2008; 15(suppl 1): S30-S40.
- Paterson A, Shea-Budgel M. Bone health in patients with breast cancer: Recommendations from an evidence-based Canadian guideline. J Clin Med 2013; 2: 283-301.
- Iranikhah M, Wilborn TW, Wensel TM, et al. Denosumab for the prevention of skeletal-related events in patients with bone metastasis from solid tumor. Pharmacother 2012; 32(3): 274-284.
- Lewiecki EM. Treatment of osteoporosis with denosumab. Maturitas 2010; 66(2): 182-186.
- Booth CM, Clemons M, Dranitsaris G, et al. Chemotherapy-induced nausea and vomiting in breast cancer patients: a prospective observational study. J Support Oncol 2007; 5(8): 374-380.
- Navari RM. Palonosetron for the prevention of chemotherapy-induced nausea and vomiting: approval and efficacy. Cancer Manag Res 2009; 1: 167-176.
- Roila F, Herrstedt J, Gralla RJ, et al. Prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: Guideline update and results of the Perugia consensus conference. Supportive Care in Cancer 2011; 19(1 Suppl): 63-65.
- Wintrobe’s Clinical Hematology (13th edition). Greer JP, Arber DA, Glader B, et al (Eds). Lippincott Williams & Wilkins, 2013.
- Botrel TE, Clark OA, Clark L, et al. Efficacy of palonosetron (PAL) compared to other serotonin inhibitors (5-HT3R) in preventing chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately or highly emetogenic (MoHE) treatment: systematic review and meta-analysis. Support Care Cancer 2011; 19(6): 823-832.
- Van den Beuken-van Everdingen MHJ, De Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007; 18: 1437-1449.
- Sun V, Borneman T, Piper B, et al. Barriers to pain assessment and management in cancer survivorship. J Cancer Surviv 2008; 2: 65-71.
- Ripamonti CI, Santini D, Maranzano E, et al. Management of Cancer Pain: ESMO Clinical Practice Guidelines. Ann Oncol 2012; 23 (Suppl 7): vii39-vii154.
- World Health Organization. Cancer Pain Relief. Geneva: World Health Organization; 1986.
- World Health Organization. Cancer Pain Relief. 2nd edition. Geneva: World Health Organization; 1996.
- Ripamonti C, Bandieri E, Roila F, on behalf of the ESMO Guidelines Working Group. Management of cancer pain: ESMO Clinical Practice Guidelines. Ann Oncol 2011; 22(Suppl 6): 69-77.
- WHO cancer pain ladder for adults. Accessed 28th March 2014. http://www.who.int/cancer/palliative/painladder/en/
- Boger RH. Renal impairment: a challenge for opioid treatment? The role of buprenorphine. Palliat Med 2006; 20: s17-s23.
- Aapro MS. Supportive care and palliative care: a time for unity in diversity. Ann Oncol 2012; 23(8): 1932-1934.